Macromolecular Chemistry
The story how human beings gained mastery over metals is well known and has been enshrined in the terms “Bronze Age” and “Iron Age”. Polymers are altogether more complex than metals, and the history of the evolution of our familiarity with polymers, from the first wooden club to the latest carbon-fibre materials, is still largely untold. This web page is an attempt to show part of that story by focussing on certain topics in polymer chemistry.
Macromolecular science covers a huge field of research, focused on the creation, the understanding, and tailoring of materials formed out of very nigh-molecular-weight molecules. These compounds – macromolecules or polymers – represent long linear chains, cyclic, branched, crosslinked, hyperbranched or dendritic architectures. Nature developed macromolecules for many specific purposes: cellulose, starch, glycogen, proteins, natural rubber, DNA… Macromolecules supplemented pr substituted classic materials (wool, leather, cotton, wood, straw, paper, metals…) due to their easy processability, availability, price and weight.
Let me introduce some interesting topics related to Macromolecular Chemistry and Soft Matter.
Thermotropic Liquid Crystals
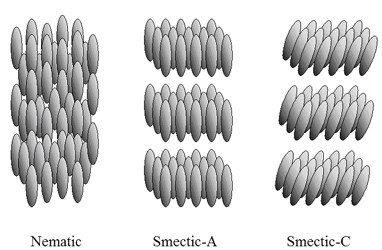
Thermotropic liquid crystals show co-operative behaviour over the temperature range just after they start to melt. To exhibit this phenomenon, a molecule needs to have a high aspect ratio: its length should be several times its cross-sectional area. Such rod-like molecules - mesogens - cannot easily tumble past each other when lattice bonds are broken at the onset of melting. A number of ordered liquid forms can occur as planes of molecules start to float apart, still keeping interplanar integrity, leading to a smectic phase (Sm). The liquid looks milky due to scattering by particles of similar dimensions to the wavelength of visible light. Further heating produces disruption on intraplanar forces, but still the molecules cannot freely tumble and hence the nematic phase (N) is formed. Last heating provides sufficient energy for tumbling to give the optical transparent isotropic liquid with random orientation of each molecule.

Nematic (N) and smectic mesophases (SmA and SmC)
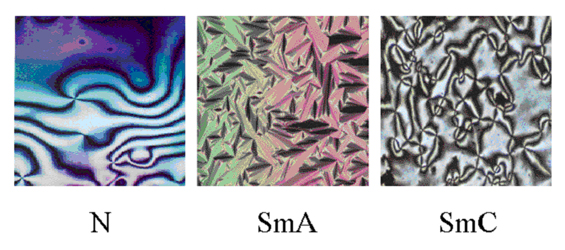
Schlieren textures of a nematic (N), and smectic-C (SmC) phases and the focal-conic fan texture of smectic-A (SmA) phase
Liquid-Crystaline Polymers
Macromolecules with liquid-crystalline moieties tend to form mesopahes, in particular when the mesogens have strong dipole moments and/or are easily polarizable. Such polymers are called liquid-crystalline polymers (LCPs). LCPs may have their rod- or disc-like mesogens either within their main chains or as lateral substituents. In main-chain LCPs the mesogens are part of the polymer backbone, while side-chain LCPs are formed when the mesogens are connected as lateral substituents to the polymer via a flexible spacer. LCPs are interesting due to their good barrier properties, high thermal stability, high modulus and strength, dimensional stability, and high chemical and solvent resistance, facts that made them useful for magnetic, electrical and optical applications.
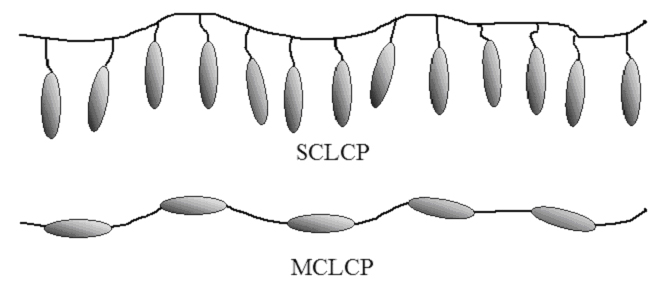
Representation of SCLCP and MCLCP
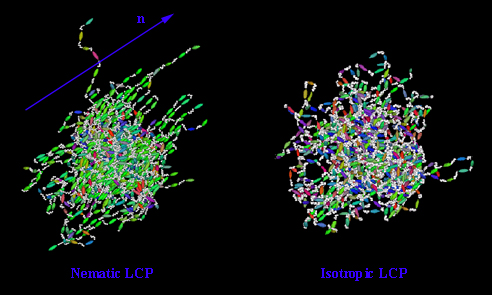
LCP in the nematic phase (N) and in the isotropic phase (I)
Liquid-Crystalline Elastomers
When multifunctional monomers are used in a polymerisation reaction, networks are obtained. Elastomers, or rubbery materials, have a loose crosslinked structure. This type of chain structure causes elastomers to possess memory. Typically, about 1 in 100 molecules are crosslinked on average. When the average number of crosslinks rises to about 1 in 30 molecules the material becomes more rigid and brittle. Thus, the degree of crosslinking determines strongly the mechanical properties of liquid-crystalline elastomers (LCEs). Depending on the curing temperature, the properties of these LCE phases can be fixed permanently.
Elastomers show rubber-like properties at ambient temperature. Their elastic moduli or Young’s moduli (E) are of the order of 104 to 106 kPa. They can be reversibly deformed without destruction, and they show very high elongations at break – more than 500%. The properties of an elastomer are determined essentially by the number of crosslinks: assumed that the glass transition temperature (Tg) is sufficiently low, weakly crosslinked elastomers are highly elastic and have a low elastic modulus. Upon increasing the crosslinking density, the elasticity decreases and the elastic modulus (E) rises.
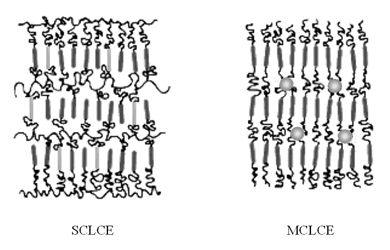
Representation of SCLCE and MCLCE
In well oriented LCEs – by imposed fields like mechanical, electric or magnetic field –, the liquid-crystalline field avoids the coil conformation due to the rubber elasticity. Then, on increasing temperature, the liquid-crystalline phase is getting destroyed and, as a consequence, the polymer tendency to form a coil shape induces the elastomer to shrink. As a result, the elastomer becomes shorter and less ordered. In the contrary process, on cooling the sample, the liquid-crystalline phase appears again and works against the entropy elasticity from the polymers. Thus, the elastomer elongates and is more ordered. This is the common scenario for LCEs when they are monodomains: an enthalpy factor from the liquid-crystalline phase against an entropy factor from the rubber elasticity.
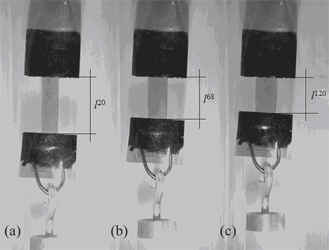
Uniaxial thermal expansion (lISO) pictures in the nematic phase at 20 ºC (a), at the clearing temperature of TNI = 68 ºC and in the isotropic phase at 120 ºC for a nematic SCLCE
Sol-Gel Technology
Sol-gel materials encompass a wide range of inorganic and organic composite materials, which share a common preparation strategy. They are prepared via sol-gel processing involving the generation of colloidal suspension – “sol” –, which is subsequently converted to viscous “gel” and thence to a solid material. Using this process films, coatings, foams, aerogels, xerogels, particles and ceramics can be obtained with a hug number of applications in different technological fields.
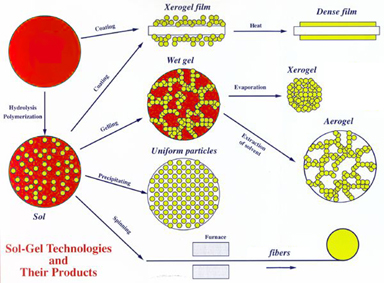 Sol-Gel
Technologies and their products
Sol-Gel
Technologies and their products
 Network by Sol-Gel Technique
Network by Sol-Gel Technique





Leo H.A. Baekeland Pierre E.M. Berthelot Wallace H. Carothers Paul J. Flory John W. Hyatt



Pierre-Gilles de Gennes Maurice L. Huggins Herman F. Mark Hermann Staudinger Carl S. Marvel
